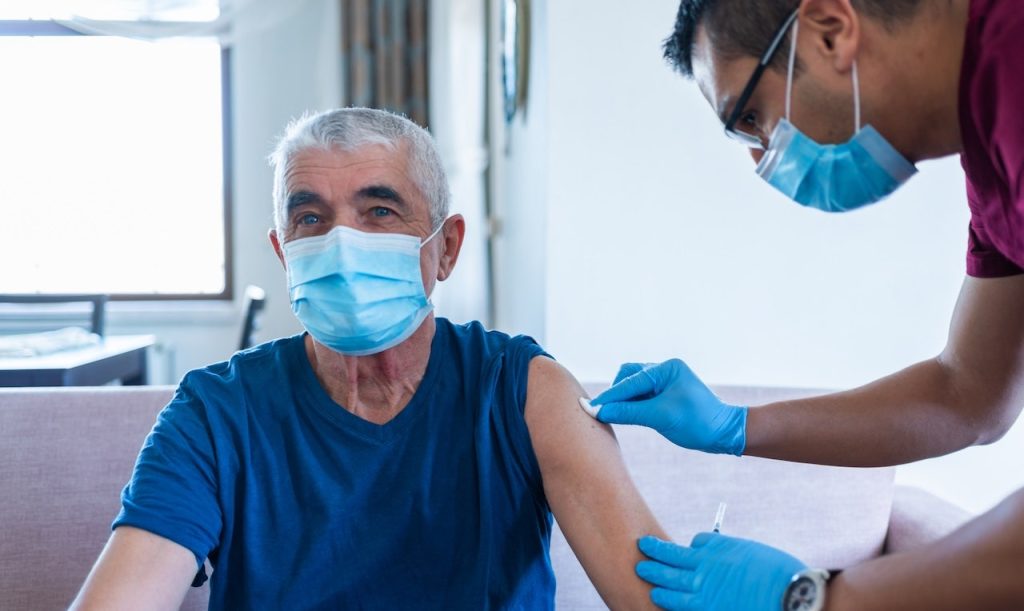
The recent approval of the Ixchiq vaccine by the Food and Drug Administration (FDA) represents a significant advancement in public health efforts to combat the chikungunya virus, a mosquito-borne disease that is rapidly emerging as a global health concern. This vaccine, approved for adults aged 18 and older, is intended to protect against the chikungunya virus, which has reportedly affected over five million people worldwide in the last 15 years. However, new recommendations from the FDA and the Centers for Disease Control and Prevention (CDC) suggest that adults over 60 should refrain from using the vaccine due to serious safety concerns.
| Article Subheadings |
|---|
| 1) Overview of the Ixchiq Vaccine and Its Approval |
| 2) Recommendations for Older Adults |
| 3) Safety Concerns and Reported Adverse Events |
| 4) Implications of Vaccination for Public Health |
| 5) Future Directions and Ongoing Assessments |
Overview of the Ixchiq Vaccine and Its Approval
The Ixchiq vaccine, developed by Valneva, was officially approved by the FDA in November 2023, marking a historic milestone in vaccine development aimed at combating chikungunya, a viral infection transmitted by Aedes mosquitoes. It stands out as the first vaccine specifically designed to provide protection against this emerging global health threat. The FDA’s approval allows the vaccine to be administered to individuals aged 18 and older who are at risk of exposure to the chikungunya virus, which predominantly affects populations residing in tropical and subtropical regions.
Chikungunya is characterized by sudden onset fever and severe joint pain, along with other symptoms such as headaches, muscle pain, and rashes. There have been increasing reports of outbreaks and cases in the Americas, influencing the urgency behind vaccine development. Public health authorities emphasize that vaccination is crucial in curbing the spread of the virus and preventing future outbreaks.
Recommendations for Older Adults
Following the vaccine’s approval, both the FDA and CDC issued a safety notice on May 9, highlighting significant concerns regarding the use of the Ixchiq vaccine in adults aged 60 and above. Due to emerging safety data indicating potential severe complications, these agencies have recommended that older adults pause using the vaccine until further evaluations are conducted. This recommendation aims to address growing concerns over the safety and efficacy of the vaccine in older populations, as their age often accompanies compromised immune responses and pre-existing health conditions.
The advisory was made particularly imperative after analyzing preliminary safety reports. It serves as a reminder to healthcare professionals to consider the risk-to-benefit ratio when administering the vaccine to older adults, who may be more susceptible to adverse outcomes. The FDA assures that they are actively monitoring safety reports in collaboration with the CDC to reassess the viability of Ixchiq for this demographic over time.
Safety Concerns and Reported Adverse Events
As concerns mount regarding the Ixchiq vaccine, the reports indicate alarming cases of “serious adverse events” among recipients, particularly within the older demographics. Early investigations revealed that two out of 17 recorded adverse events led to fatalities. One incident involved neurological complications, specifically encephalitis, which presents as inflammation in the brain and can have devastating outcomes.
The age range of affected individuals reported adverse effects varied between 62 to 89 years. These complications have prompted regulatory agencies to issue strong warnings about potential risks associated with the vaccine. While serious side effects have been noted, it is essential to recognize that adverse events were not uniformly observed across all individuals, and most reports point to rare occurrences.
Implications of Vaccination for Public Health
Despite the safety concerns surrounding the Ixchiq vaccine for older adults, the vaccine’s approval is a significant step in addressing public health needs, especially in regions prone to chikungunya outbreaks. The potential for the vaccine to reduce the incidence of chikungunya infections grounds its importance in global vaccination strategies. Public health experts warn that the chikungunya virus, prevalent in many areas including Latin America and parts of Asia, could have far-reaching implications if not adequately controlled.
Dr. Marc Siegel, a senior medical analyst, notes that chikungunya is in a similar category to dengue and Zika, both conveyed through similar vectors, and thus highlights the necessity for the vaccine as a public health measure. The increase in these diseases prompts concerted efforts amongst healthcare institutions to prioritize vaccine dissemination while maintaining safety protocols. This balance is crucial for fostering public trust in vaccination campaigns aimed at controlling not just chikungunya but other vector-borne diseases.
Future Directions and Ongoing Assessments
Looking ahead, the FDA has committed to conducting an updated benefit-risk assessment of the Ixchiq vaccine, specifically focusing on its safety for older adults. This systematic review will involve reevaluating the post-marketing safety data, where more information will emerge as the vaccination efforts progress. The agencies plan to update guidelines based on this ongoing evaluation to ensure that any vaccine administered remains safe and effective.
Public health authorities continue to encourage healthcare providers to remain vigilant and educate patients regarding the emerging safety concerns, particularly for older populations with comorbid conditions that place them at increased risk. These efforts will aid in establishing a long-term strategy for chikungunya vaccination, which is paramount given the significant rise in reported cases globally.
| No. | Key Points |
|---|---|
| 1 | The Ixchiq vaccine has been officially approved by the FDA for adults 18 and older. |
| 2 | FDA and CDC recommend older adults pause receiving the vaccine due to safety concerns. |
| 3 | Reports of serious adverse events, including neurological issues, have emerged following vaccination. |
| 4 | Public health experts emphasize the importance of the vaccine to curb chikungunya outbreaks. |
| 5 | Ongoing assessments by FDA will further evaluate Ixchiq’s safety profile for older adults. |
Summary
The approval of the Ixchiq vaccine marks a notable advancement against the chikungunya virus, a disease increasingly posing risks to global health. Nonetheless, the recent safety notifications for older adults underline the complexities involved in vaccine administration, particularly for vulnerable populations. As regulatory agencies continue evaluating the safety profile of Ixchiq, the commitment to balancing effective vaccination against emerging health threats remains paramount.
Frequently Asked Questions
Question: What is the Ixchiq vaccine used for?
The Ixchiq vaccine is designed to protect against the chikungunya virus, a mosquito-borne disease that can cause severe symptoms, including high fever and debilitating joint pain.
Question: Why are older adults advised to pause vaccination with Ixchiq?
Older adults are advised to pause vaccination due to emerging safety data indicating serious adverse events, including severe neurological and cardiac complications.
Question: What symptoms does chikungunya typically present?
Common symptoms of chikungunya include fever, severe joint pain, headache, muscle pain, and rashes, with some individuals experiencing long-term joint pain after recovery.